'Retrovirology' redirects here. For the journal, see.RetrovirusesHIV retrovirus schematic of cell infection, virus production and virus structure(unranked):Phylum:Class:Order:Family:RetroviridaeSubfamilies and genera.A retrovirus is a type of that inserts a copy of its into the of a that it invades, thus changing the of that cell. Once inside the host cell's, the virus uses its own enzyme to produce from its genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then into the host cell by an enzyme, at which point the retroviral DNA is referred to as a. The host cell then treats the viral DNA as part of its own, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus.Human retroviruses include and, the cause of the disease. Retroviruses are also a valuable research tools in molecular biology, and they have been used successfully in gene delivery systems.
Retrovirus meaning: a type of virus that includes some cancer viruses and HIV (= the virus that causes AIDS, a serious. Cambridge Dictionary Plus. NCI Dictionary of Cancer Terms. Retrovirus listen (REH-troh-VY-rus) A type of virus that has RNA instead of DNA as its genetic material. It uses an enzyme called reverse transcriptase to become part of the host cells’ DNA. This allows many copies of the virus to be made in the host cells.
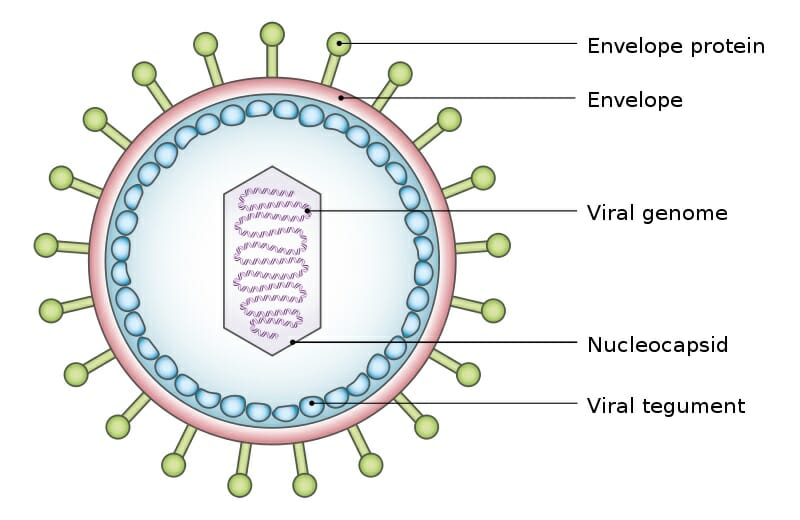
Contents.Structure of retroviruses consist of enveloped particles about 100 nm in diameter. The virions also contain two identical single-stranded molecules 7–10 in length. Although virions of different retroviruses do not have the same or biology, all the virion components are very similar.The main virion components are:.: composed of lipids (obtained from the host during the process) as well as glycoprotein encoded by the.
The retroviral envelope serves three distinct functions: protection from the extracellular environment via the, enabling the retrovirus to enter/exit host cells through, and the ability to directly enter cells by fusing with their membranes.: consists of a RNA. It has a cap at the end and a poly(A) tail at the 3' end. The RNA genome also has terminal noncoding regions, which are important in replication, and internal regions that encode proteins for. The 5' end includes four regions, which are R, U5, PBS, and L.
The R region is a short repeated sequence at each end of the genome used during the to ensure correct end-to-end transfer in the growing chain. U5, on the other hand, is a short unique sequence between R and PBS.
PBS (primer binding site) consists of 18 bases complementary to 3' end of tRNA primer. L region is an untranslated leader region that gives the signal for packaging of the genome RNA. The 3' end includes 3 regions, which are PPT (polypurine tract), U3, and R. The PPT is a primer for plus-strand DNA synthesis during. U3 is a sequence between PPT and R, which serves as a signal that the provirus can use in. R is the terminal repeated sequence at 3' end.: consisting of gag proteins, (PR), pol proteins, and env proteins.
(gag) proteins are major components of the viral, which are about 2000–4000 copies per virion. (pro) is expressed differently in different viruses.
It functions in proteolytic cleavages during virion maturation to make mature gag and pol proteins. are responsible for synthesis of viral DNA and integration into host DNA after infection. play a role in association and entry of virions into the host cell. Possessing a functional copy of an is what makes retroviruses distinct from. The ability of the retrovirus to bind to its target host cell using specific cell-surface receptors is given by the surface component (SU) of the Env protein, while the ability of the retrovirus to enter the cell via is imparted by the membrane-anchored trans-membrane component (TM).
Thus it is the Env protein that enables the retrovirus to be infectious.Genomic structure. The genomic and subgenomic organization of a prototypical retrovirus. Abbreviations are explained in the file description.Retroviruses (and in general) follow a layout of 5'- gag- pro- pol- env-3' in the RNA genome. Gag and pol encode polyproteins, each managing the capsid and replication. Depending on the virus, the genes may overlap or fuse into larger polyprotein chains. Some viruses contain additional genes.The polyproteins are cleaved into smaller proteins each with their own function. The nucleotides encoding them are known as subgenes.
Multiplication. A retrovirus has a membrane containing glycoproteins, which are able to bind to a receptor protein on a host cell. There are two strands of RNA within the cell that have three enzymes: protease, reverse transcriptase, and integrase (1). The first step of replication is the binding of the glycoprotein to the receptor protein (2).
Once these have been bound, the cell membrane degrades, becoming part of the host cell, and the RNA strands and enzymes enter the cell (3). Within the cell, reverse transcriptase creates a complementary strand of DNA from the retrovirus RNA and the RNA is degraded; this strand of DNA is known as cDNA (4). The cDNA is then replicated, and the two strands form a weak bond and enter the nucleus (5). Once in the nucleus, the DNA is integrated into the host cell's DNA with the help of integrase (6).
This cell can either stay dormant, or RNA may be synthesized from the DNA and used to create the proteins for a new retrovirus (7). Ribosome units are used to translate the mRNA of the virus into the amino acid sequences which can be made into proteins in the rough endoplasmic reticulum. This step will also make viral enzymes and capsid proteins (8). Viral RNA will be made in the nucleus. These pieces are then gathered together and are pinched off of the cell membrane as a new retrovirus (9).When retroviruses have integrated their own genome into the, their genome is to a following generation. These (ERVs), contrasted with ones, now make up 5-8% of the human genome. Most insertions have no known function and are often referred to as '.
However, many endogenous retroviruses play important roles in host biology, such as control of gene transcription, cell fusion during development in the course of the of an, and resistance to exogenous retroviral infection. Endogenous retroviruses have also received special attention in the research of -related pathologies, such as like, although endogenous retroviruses have not yet been proven to play any causal role in this class of disease.While transcription was classically thought to occur only from DNA to RNA, transcribes RNA into DNA. The term 'retro' in retrovirus refers to this reversal (making DNA from RNA) of the usual direction of transcription. It still obeys the, which states that information can be transferred from nucleic acid to nucleic acid but cannot be transferred back from protein to either protein or nucleic acid.
Reverse transcriptase activity outside of retroviruses has been found in almost all, enabling the generation and insertion of new copies of into the host genome. These inserts are transcribed by enzymes of the host into new RNA molecules that enter the cytosol. Next, some of these RNA molecules are translated into viral proteins. For example, the gag gene is translated into molecules of the capsid protein, the pol gene is translated into molecules of reverse transcriptase, and the env gene is translated into molecules of the envelope protein. It is important to note that a retrovirus must 'bring' its own reverse transcriptase in its, otherwise it is unable to utilize the enzymes of the infected cell to carry out the task, due to the unusual nature of producing DNA from RNA.Industrial drugs that are designed as protease and are made such that they target specific sites and sequences within their respective enzymes. However these drugs can quickly become ineffective due to the fact that the gene sequences that code for the protease and the reverse transcriptase quickly mutate.
These changes in bases cause specific codons and sites with the enzymes to change and thereby avoid drug targeting by losing the sites that the drug actually targets.Because reverse transcription lacks the usual of DNA replication, a retrovirus very often. This enables the virus to grow to antiviral pharmaceuticals quickly, and impedes the development of effective and inhibitors for the retrovirus.One difficulty faced with some retroviruses, such as the Moloney retrovirus, involves the requirement for cells to be actively dividing for transduction. As a result, cells such as neurons are very resistant to infection and transduction by retroviruses. This gives rise to a concern that insertional mutagenesis due to integration into the host genome might lead to cancer or leukemia.
This is unlike, a genus of Retroviridae, which are able to integrate their RNA into the genome of non-dividing host cells.Transmission. Cell-to-cell. Fluids. Airborne, like the.Provirus This DNA can be incorporated into host genome as a provirus that can be passed on to progeny cells.
The retrovirus DNA is inserted at random into the host genome. Because of this, it can be inserted into. In this way some retroviruses can convert normal cells into cancer cells. Some provirus remains latent in the cell for a long period of time before it is activated by the change in cell environment.Early evolution Studies of retroviruses led to the first demonstrated synthesis of DNA from RNA templates, a fundamental mode for transferring genetic material that occurs in both. It has been speculated that the RNA to DNA transcription processes used by retroviruses may have first caused DNA to be used as genetic material. In this model, the, cellular organisms adopted the more chemically stable DNA when retroviruses evolved to create from the templates.An estimate of the date of evolution of the foamy-like endogenous retroviruses placed the time of the most recent common ancestor at million years ago. Gene therapy and for have been developed that mediate stable genetic modification of treated cells by chromosomal integration of the transferred vector genomes.
This technology is of use, not only for research purposes, but also for clinical gene therapy aiming at the long-term correction of genetic defects, e.g., in stem and progenitor cells. Retroviral vector particles with tropism for various target cells have been designed. Gammaretroviral and lentiviral vectors have so far been used in more than 300 clinical trials, addressing treatment options for various diseases.
Retroviral mutations can be developed to make transgenic mouse models to study various cancers and their.Cancer Retroviruses that cause tumor growth include. Cancer can be triggered by proto-oncogenes that were mistakenly incorporated into proviral DNA or by the disruption of cellular proto-oncogenes. Rous sarcoma virus contains the that triggers tumor formation.
Later it was found that a similar gene in cells is involved in cell signaling, which was most likely excised with the proviral DNA. Nontransforming viruses can randomly insert their DNA into proto-oncogenes, disrupting the expression of proteins that regulate the cell cycle. The promoter of the provirus DNA can also cause over expression of regulatory genes.Classification. Phylogeny of Retroviruses Exogenous These are infectious RNA- or DNA-containing viruses which are transmitted from person to person.Reverse-transcribing viruses fall into 2 groups of the.
Group VI viruses All members of use virally encoded, an RNA-dependent DNA polymerase, to produce DNA from the initial virion RNA genome. This DNA is often integrated into the host genome, as in the case of retroviruses and, where it is replicated and by the host.Group VI includes:. Order. Family. Family.
Family. Family – Retroviruses, e.g. Family – a VII group virus family (see below)The family Retroviridae was previously divided into three subfamilies ( Oncovirinae, Lentivirinae, and Spumavirinae), but are now divided into two: Orthoretrovirinae and Spumaretrovirinae. The term is now commonly used to describe a cancer-causing virus. Main article:Both families in have DNA genomes contained within the invading virus particles. The DNA genome is transcribed into both mRNA, for use as a transcript in protein synthesis, and pre-genomic RNA, for use as the template during genome replication.
Virally encoded uses the pre-genomic RNA as a template for the creation of genomic DNA.Group VII includes:. Family — e.g. Family — e.g.The latter family is closely related to the newly proposed. Family — e.g. (ACNDV), formerly named African cichlid hepatitis B virus (ACHBV).whilst families, and constitute the order. Endogenous.
Main article:Endogenous retroviruses are not formally included in this classification system, and are broadly classified into three classes, on the basis of relatedness to exogenous genera:. Class I are most similar to the gammaretroviruses. Class II are most similar to the betaretroviruses and alpharetroviruses. Class III are most similar to the spumaviruses.Treatment are medications for the treatment of infection by retroviruses, primarily. Different classes of antiretroviral drugs act on different stages of the HIV. Combination of several (typically three or four) antiretroviral drugs is known as highly active anti-retroviral therapy (HAART). Treatment of veterinary retroviruses and infections are treated with, including the only immunomodulator currently licensed for sale in the United States, (LTCI).
Of various classes of endogenous retrovirusesEndogenous retroviruses ( ERVs) are in the that closely resemble and can be derived from. They are abundant in the genomes of, and they comprise up to 5–8% of the human genome (lower estimates of 1%). ERVs are a subclass of a type of called a, which can be packaged and moved within the genome to serve a vital role in and in. They are distinguished as, which are Class I elements. Researchers have suggested that retroviruses evolved from a type of called a, which includes ERVs; these genes can mutate and instead of moving to another location in the genome they can become exogenous or pathogenic. This means that not all ERVs may have originated as an insertion by a retrovirus but that some may have been the source for the genetic information in the retroviruses they resemble. When integration of viral DNA occurs in the germ-line, it can give rise to an ERV, which can later become fixed in the gene pool of the host population.
Contents.Formation The replication cycle of a entails the insertion ('integration') of a DNA copy of the viral into the genome of the. Most retroviruses infect, but occasional infection of cells (cells that produce eggs and sperm) can also occur. Rarely, retroviral integration may occur in a germline cell that goes on to develop into a viable organism. This organism will carry the inserted retroviral genome as an integral part of its own genome—an 'endogenous' retrovirus (ERV) that may be by its as a novel. Many ERVs have persisted in the genome of their hosts for millions of years.
However, most of these have acquired inactivating during host and are no longer capable of producing the virus. ERVs can also be partially excised from the genome by a process known as recombinational deletion, in which between the identical sequences that flank newly integrated retroviruses results in deletion of the internal, -coding regions of the viral genome.The general retrovirus genome consists of three genes vital for the invasion, replication, escape, and spreading of its viral genome. These three genes are gag (encodes for structural proteins for the viral core), pol (encodes for, and ), and env (encodes for for the virus's exterior). These viral proteins are encoded as. In order to carry out their life cycle, the retrovirus relies heavily on the host cell's machinery.
Protease degrades peptide bonds of the viral polyproteins, making the separate proteins functional. Reverse transcriptase functions to synthesize viral DNA from the viral RNA in the host cell's cytoplasm before it enters the nucleus. Integrase guides the integration of viral DNA into the host genome.Over time, the genome of ERVs not only acquire point mutations, but also shuffle and recombine with other ERVs. ERVs with the env coat decayed become more likely to spread. Role in genome evolution. Endogenous retroviruses can play an active role in shaping genomes.
Most studies in this area have focused on the genomes of humans and higher primates, but other vertebrates, such as mice and sheep, have also been studied in depth. The long terminal repeat sequences that flank ERV genomes frequently act as alternate and, often contributing to the by producing tissue-specific variants. In addition, the retroviral themselves have been co-opted to serve novel host functions, particularly in reproduction and development. Recombination between homologous retroviral sequences has also contributed to gene shuffling and the generation of genetic variation.
Furthermore, in the instance of potentially antagonistic effects of retroviral sequences, repressor genes have co-evolved to combat them.Solo LTRs and LTRs associated with complete retroviral sequences have been shown to act as transcriptional elements on host genes. Their range of action is mainly by insertion into the 5' of protein coding genes; however, they have been known to act upon genes up to 70–100 away. The majority of these elements are inserted in the sense direction to their corresponding genes, but there has been evidence of LTRs acting in the antisense direction and as a bidirectional promoter for neighboring genes. In a few cases, the LTR functions as the major promoter for the gene.
For example, in humans AMY1C has a complete ERV sequence in its promoter region; the associated LTR confers salivary specific expression of the digestive. Also, the primary promoter for (BAAT), which codes for an enzyme that is integral in bile metabolism, is of LTR origin.The insertion of a solo ERV-9 LTR may have produced a functional (ORF), causing the rebirth of the human (IRGM). ERV insertions have also been shown to generate alternative splice sites either by direct integration into the gene, as with the human leptin hormone receptor, or driven by the expression of an upstream LTR, as with the phospholipase A-2 like protein.Most of the time, however, the LTR functions as one of many alternate promoters, often conferring tissue-specific expression related to reproduction and development. In fact, 64% of known LTR-promoted transcription variants are in reproductive tissues. For example, the gene CYP19 codes for P450, an important enzyme for estrogen synthesis, that is normally expressed in the brain and reproductive organs of most mammals. However, in primates, an LTR-promoted transcriptional variant confers expression to the placenta and is responsible for controlling estrogen levels during pregnancy.
Furthermore, the (NAIP), normally widespread, has an LTR of the HERV-P family acting as a promoter that confers expression to the testis and prostate. Other proteins, such as nitric oxide synthase 3 (NOS3), interleukin-2 receptor B (IL2RB), and another mediator of estrogen synthesis, HSD17B1, are also alternatively regulated by LTRs that confer placental expression, but their specific functions are not yet known. The high degree of reproductive expression is thought to be an after effect of the method by which they were endogenized; however, this also may be due to a lack of DNA methylation in germ-line tissues.The best-characterized instance of placental protein expression comes not from an alternatively promoted host gene but from a complete co-option of a retroviral protein. Retroviral fusogenic env proteins, which play a role in the entry of the virion into the host cell, have had an important impact on the development of the mammalian. In mammals, intact env proteins called are responsible for the formation and function of. These multinucleated cells are mainly responsible for maintaining nutrient exchange and separating the fetus from the mother's immune system.
It has been suggested that the selection and fixation of these proteins for this function have played a critical role in the evolution of.In addition, the insertion of ERVs and their respective LTRs have the potential to induce chromosomal rearrangement due to recombination between viral sequences at inter-chromosomal loci. These rearrangements have been shown to induce gene duplications and deletions that largely contribute to genome plasticity and dramatically change the dynamic of gene function. Furthermore, retroelements in general are largely prevalent in rapidly evolving, mammal-specific gene families whose function is largely related to the response to stress and external stimuli. In particular, both human and genes have a high density of HERV elements as compared to other multi-locus-gene families. It has been shown that HERVs have contributed to the formation of extensively duplicated duplicon blocks that make up the HLA class 1 family of genes. More specifically, HERVs primarily occupy regions within and between the break points between these blocks, suggesting that considerable duplication and deletions events, typically associated with unequal crossover, facilitated their formation. The generation of these blocks, inherited as immunohaplotypes, act as a protective polymorphism against a wide range of antigens that may have imbued humans with an advantage over other primates.Finally, the insertion of ERVs or ERV elements into genic regions of host DNA, or overexpression of their transcriptional variants, has a much higher potential to produce deleterious effects than positive ones.
Their appearance into the genome has created a that proliferated the duplication and expansion of repressor genes. The most clear-cut example of this involves the rapid duplication and proliferation of tandem genes in mammal genomes. Zinc-finger genes, particularly those that include a KRAB domain, exist in high copy number in vertebrate genomes, and their range of functions are limited to transcriptional roles.
It has been shown in mammals, however, that the diversification of these genes was due to multiple duplication and fixation events in response to new retroviral sequences or their endogenous copies to repress their transcription. The characteristic of being very evolutionary distinct organs between different species has been suggested to result from the of ERV enhancers. Regulatory mutations, instead of mutations in genes that encode for and, support the known evolution of placental morphology, especially since the majority of hormone and growth factor genes are expressed in response to pregnancy, not during placental development. Researchers studied the regulatory landscape of placental development between the rat and mouse, two closely related species. This was done by mapping all regulatory elements of the rat s (TSCs) and comparing them to their in mouse TSCs. TSCs were observed because they reflect the initial cells that develop in the fetal placenta.
Regardless of their tangible similarities, enhancer and repressed regions were mostly species-specific. However, most promoter sequences were conserved between mouse and rat. In conclusion to their study, researchers proposed that ERVs influenced species-specific placental evolution through mediation of placental growth, and.Another example of ERV exploiting cellular mechanisms is, a (TSG). DNA damage and cellular stress induces the p53 pathway, which results in cell. Using chromatin immunoprecipitation with sequencing, thirty-percent of all p53-binding sites were located within copies of a few primate-specific ERV families.
A study suggested that this benefits retroviruses because p53's mechanism provides a rapid induction of transcription, which leads to the exit of viral RNA from the host cell. Role in disease The majority of ERVs that occur in vertebrate genomes are ancient, inactivated by mutation, and have reached genetic in their host species.
For these reasons, they are extremely unlikely to have negative effects on their hosts except under unusual circumstances. Nevertheless, it is clear from studies in birds and non-human mammal species including mice, cats and, that younger (i.e., more recently integrated) ERVs can be associated with disease. The number of active ERVs in the genome of mammals is negatively related to their body size suggesting a contribution to the through cancer pathogenesis. This has led researchers to propose a role for ERVs in several forms of human cancer and, although conclusive evidence is lacking. Neurological disorders In humans, ERVs have been proposed to be involved in (MS).
A specific association between MS and the, or 'syncytin', gene, which is derived from an ERV insertion, has been reported, along with the presence of an 'MS-associated retrovirus' (MSRV), in patients with the disease. Human ERVs (HERVs) have also been implicated in and addiction.In 2004 it was reported that antibodies to HERVs were found in greater frequency in the of people with. Additionally, the of people with recent onset schizophrenia contained levels of a retroviral marker, four times higher than control subjects. Researchers continue to look at a possible link between HERVs and schizophrenia, with the additional possibility of a triggering. Immunity ERVs have been found to be associated to disease not only through disease-causing relations, but also through immunity.
The frequency of ERVs in long terminal repeats (LTRs) likely correlates to viral adaptations to take advantage of immunity signaling pathways that promote viral transcription and replication. A study done in 2016 investigated the benefit of ancient viral DNA integrated into a host through gene regulation networks induced by, a branch of innate immunity.
These are first to respond to viral infection and are also important in immunosurveillance for malignant cells. ERVs are predicted to act as cis-regulatory elements, but much of the adaptive consequences of this for certain physiological functions is still unknown. There is data that supports the general role of ERVs in the regulation of human interferon response, specifically to (IFNG). For example, interferon-stimulated genes were found to be greatly enriched with ERVs bound by signal transducer and activator of transcription (STAT1) and/or (IRF1) in macrophages.HERVs also play various roles shaping the human response, with some sequences activating the system and others suppressing it. They may also protect from exogenous retroviral infections: the virus-like transcripts can activate, and the proteins can interfere with active retroviruses. A gag protein from HERV-K(HML2) is shown to mix with HIV Gag, impairing HIV capsid formation as a result. Gene regulation Another idea proposed was that ERVs from the same family played a role in recruiting multiple genes into the same network of regulation.
It was found that MER41 elements provided addition redundant regulatory enhancement to the genes located near STAT1 binding sites. Role in medicine Porcine endogenous retrovirus. See also:For humans, endogenous retroviruses (PERVs) pose a concern when using porcine tissues and organs in xenotransplantion, the transplanting of living cells, tissues, and organs from an organism of one species to an organism of different species. Although pigs are generally the most suitable donors to treat human organ diseases due to practical, financial, safety, and ethical reasons, PERVs previously could not be removed from pigs due to its viral nature of integrating into the host genome and being passed into offspring until the year 2017 when Dr. George Church's lab removed all 62 retroviruses from the pigs genome. The consequences of cross-species transmission remains unexplored and has very dangerous potential.Researchers indicated that infection of human tissues by PERVs is very possible, especially in immunosuppressed individuals.
An immunosuppressed condition could potentially permit a more rapid and tenacious replication of viral DNA, and would later on have less difficulty adapting to human-to-human transmission. Although known infectious pathogens present in the donor organ/tissue can be eliminated by breeding pathogen-free herds, unknown retroviruses can be present in the donor. These retroviruses are often latent and asymptomatic in the donor, but can become active in the recipient. Some examples of endogenous viruses that can infect and multiply in human cells are from baboons (BaEV), cats (RD114), and mice.There are three different classes of PERVs, PERV-A, PERV-B, and PERV-C. PERV-A and PERV-B are and can infect human cells in vitro, while PERV-C is and does not replicate on human cells.
The major differences between the classes is in the receptor binding domain of the env protein and the long terminal repeats (LTRs) that influence the replication of each class. PERV-A and PERV-B display LTRs that have repeats in the region. However, PERV-A and PERV-C show repeatless LTRs.
Researchers found that PERVs in culture actively adapted to the repeat structure of their LTR in order to match the best replication performance a host cell could perform. At the end of their study, researchers concluded that repeatless PERV LTR evolved from the repeat-harboring LTR. This was likely to have occurred from insertional mutation and was proven through use of data on LTR and env/Env.
It is thought that the generation of repeatless LTRs could be reflective of an adaptation process of the virus, changing from an exogenous to an endogenous lifestyle.A clinical trial study performed in 1999 sampled 160 patients who were treated with different living pig tissues and observed no evidence of a persistent PERV infection in 97% of the patients for whom a sufficient amount of DNA was available to PCR for amplification of PERV sequences. This study stated that retrospective studies are limited to find the true incidence of infection or associated clinical symptoms, however. It suggested using closely monitored prospective trials, which would provide a more complete and detailed evaluation of the possible cross-species PERV transmission and a comparison of the PERV.
Human endogenous retroviruses. 'HERV' redirects here. For other uses, see.Human endogenous retroviruses (HERV) comprise a significant part of the, with approximately 98,000 ERV elements and fragments making up 5–8%. According to a study published in 2005, no HERVs capable of replication had been identified; all appeared to be defective, containing major deletions or nonsense mutations. This is because most HERVs are merely traces of original viruses, having first integrated millions of years ago.
An analysis of HERV integrations is ongoing as part of the.Human endogenous retroviruses were discovered by accident using a couple of different experiments. Human genomic libraries were screened under low-stringency conditions using probes from animal retroviruses, allowing the isolation and characterization of multiple, though defective, proviruses, that represented various families.
Another experiment depended on oligonucleotides with homology to viral primer binding sites.HERVs are classified based on their homologies to animal retroviruses. Families belong to Class I are similar in sequence to mammalian (type C) and (Type E). Families belonging to Class II show homology to mammalian (Type B) and (Type D). Families belong to Class III are similar to. For all classes, if homologies appear well conserved in the gag, pol, and env gene, they are grouped into a. There are more Class I families known to exist. Sonic and the black knight download. The families themselves are named in a less uniform manner, with a mixture of naming based on an exogenous retrovirus, the priming tRNA (HERV-W, K), or some neiboring gene (HERV-ADP), clong number (HERV-S71), or some amino acid motif (HERV-FRD).
A proposed nomenclature aims to clean up the sometimes paraphyletic standards.There are two proposals for how HERVs became fixed in the human genome. The first assumes that sometime during human evolution, exogenous progenitors of HERV inserted themselves into germ line cells and then replicated along with the host's genes using and exploiting the host's cellular mechanisms. Because of their distinct genomic structure, HERVs were subjected to many rounds of amplification and transposition, which lead to a widespread distribution of retroviral DNA. The second hypothesis claims the continuous evolution of retro-elements from more simple structured ancestors.Nevertheless, one family of viruses has been active since the divergence of. This family, termed (HML2), makes up less than 1% of HERV elements but is one of the most studied.
There are indications it has even been active in the past few hundred thousand years, e.g., some human individuals carry more copies of HML2 than others. Traditionally, age estimates of HERVs are performed by comparing the 5' and 3' of a HERV; however, this method is only relevant for full-length HERVs. A recent method, called cross-sectional dating, uses variations within a single LTR to estimate the ages of HERV insertions. This method is more precise in estimating HERV ages and can be used for any HERV insertions. Cross-sectional dating has been used to suggest that two members of HERV-K(HML2), HERV-K106 and HERV-K116, were active in the last 800,000 years and that HERV-K106 may have infected modern humans 150,000 years ago.
However, the absence of known infectious members of the HERV-K(HML2) family, and the lack of elements with a full coding potential within the published human genome sequence, suggests to some that the family is less likely to be active at present. In 2006 and 2007, researchers working independently in France and the US recreated functional versions of HERV-K(HML2).MER41.AIM2 is an HERV that regulates the transcription of AIM2 (Absent in Melanoma 2) which encodes for a sensor of foreign cytosolic DNA. This acts as a binding site for AIM2, meaning that it is necessary for the transcription of AIM2.
Researchers had shown this by deleting MER41.AIM2 in HeLa cells using CRISPR/Cas9, leading to an undetectable transcript level of AIM2 in modified HeLa cells. The control cells, which still contained the MER41.AIM2 ERV, were observed with normal amounts of AIM2 transcript. In terms of immunity, researchers concluded that MER41.AIM2 is necessary for an inflammatory response to infection.Immunological studies have shown some evidence for immune responses against HERVs in HIV-infected individuals. The hypothesis that HIV induces HERV expression in HIV-infected cells led to the proposal that a vaccine targeting HERV antigens could specifically eliminate HIV-infected cells. The potential advantage of this novel approach is that, by using HERV antigens as surrogate markers of HIV-infected cells, it could circumvent the difficulty inherent in directly targeting notoriously diverse and fast-mutating HIV antigens.There are a few classes of human endogenous retroviruses that still have intact open reading frames. For example, the expression of HERV-K, a biologically active family of HERV, produces proteins found in placenta.
Furthermore, the expression of the envelope genes of and produces syncytins which are important for the generation of the syncytiotrophoblast cell layer during placentogenesis by inducing cell-cell fusion. The (HGNC) approves gene symbols for transcribed human ERVs.
Techniques for characterizing ERVs Whole genome sequencing Example: A porcine ERV (PERV) Chinese-born isolate, PERV-A-BM, was sequenced completely and along with different breeds and cell lines in order to understand its genetic variation and evolution. The observed number of nucleotide substitutions and among the different genome sequences helped researchers determine an estimate age that PERV-A-BM was integrated into its host genome, which was found to be of an evolutionary age earlier than the European-born pigs isolates. Chromatin Immunoprecipitation with sequencing (ChIP-seq) This technique is used to find histone marks indicative of promoters and enhancers, which are binding sites for DNA proteins, and repressed regions and trimethylation. DNA methylation has been shown to be vital to maintain silencing of ERVs in mouse somatic cells, while histone marks are vital for the same purpose in (ESCs) and early embryogenesis. Applications Constructing phylogenies Because most HERVs have no function, are selectively neutral, and are very abundant in primate genomes, they easily serve as phylogenetic markers for linkage analysis. They can be exploited by comparing the integration site polymorphisms or the evolving, proviral, nucleotide sequences of orthologs. To estimate when integration occurred, researchers used distances from each phylogenetic tree to find the rate of molecular evolution at each particular locus.
It is also useful that ERVs are rich in many species genomes (i.e. Plants, insects, mollusks, fish, rodents, domestic pets, and livestock) because its application can be used to answer a variety of phylogenetic questions.
Designating the age of provirus and the time points of species separation events This is accomplished by comparing the different HERV from different evolutionary periods. For example, this study was done for different hominoids, which ranged from humans to apes and to monkeys. This is difficult to do with PERV because of the large diversity present. Further research Epigenetic variability Researchers could analyze individual epigenomes and to study the reactivation of dormant transposable elements through epigenetic release and their potential associations with human disease and exploring the specifics of gene regulatory networks.
Immunological problems of xenotransplantation Little is known about an effective way to overcoming (HAR), which follows the activation of complement initiated by xenoreactive antibodies recognizing galactosyl-alpha1-3galatosyl (alpha-Gal) antigens on the donor epithelium. Risk factors of HERVs in gene therapy Because retroviruses are able to recombine with each other and with other endogenous DNA sequences, it would be beneficial for gene therapy to explore the potential risks HERVs can cause, if any. Also, this ability of HERVs to recombine can be manipulated for site-directed integration by including HERV sequences in retroviral vectors.
HERV gene expression Researchers believe that RNA and proteins encoded for by HERV genes should continue to be explored for putative function in cell physiology and in pathological conditions. This would make sense to examine in order to more deeply define the biological significance of the proteins synthesized. See also.